A Novel Paradigm for Immuno-Oncology Therapeutics: Overcoming Low Response Rates
Bispecific T-Cell Engager
A bispecific antibody is an engineered antibody that fuses the functions of two different antibodies into one antibody. This innovative technology goes beyond simply increasing the efficiency, as in combination therapies, and enables a novel mechanism of action.
When the functions of an antibody that selectively binds to cancer cells and an antibody that activates immune cells are combined within a single antibody, it is possible to induce activation of immune cells specifically around cancer cells through the administration of a therapeutic injection.
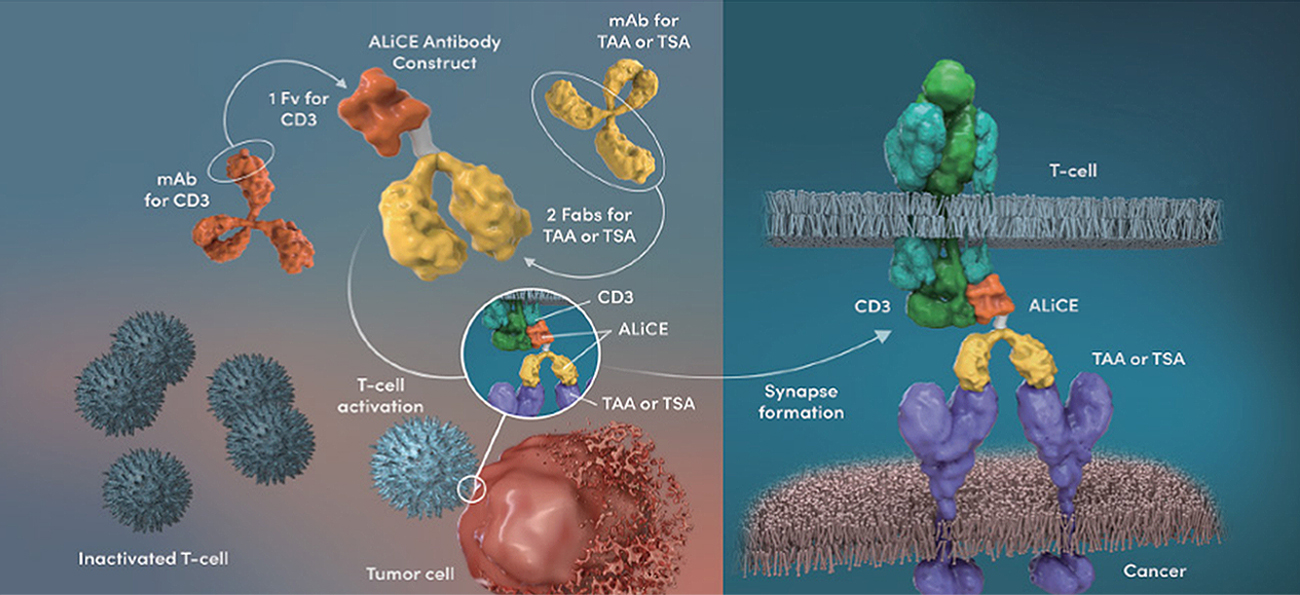
ALiCE, produced in a 2-by-1 format, is designed to mainly target the surface of cancer cells in the body, as the tumor antigen-binding titers were increased to levels much higher than that of the CD3 antibody. Also, by lowering the binding affinity of the CD3 antibody, excessive T cell activation in the blood has been minimized.
Antibody Like Cell Engager (ALiCE): Our Proprietary Bispecific T Cell Engager Structure that Binds Bivalently to Antigens on Cancer Cells and Monovalently to T Cells
As a typical Y-shaped IgG-type bispecific antibody, antibody modification based on structural engineering is minimized, making ALiCE a platform technology with the most natural bispecific antibody structure.
The upper portion of ALiCE, which maintains the structure of general antibodies, consists of bivalent Fab regions that recognize a target antigen on cancer cells. The crystallizable fragment (Fc) region of the antibody, however, is substituted with a monovalent variable fragment (Fv) region from a CD3-targeting antibody
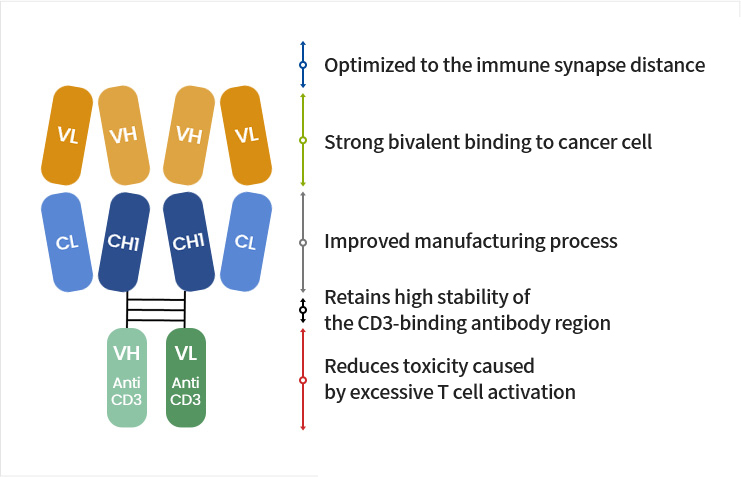
- Maintains our company’s excellent antibody profile
- Enables a difference in binding avidity (cancer cells > T cells)
- Minimizes side effects
- Excellent immunotherapeutic effect
- Minimizes side effects by activating T cells around cancer cells
Satisfying Unmet Medical Needs by Preventing Immune Escape and Specifically Recognizing and Killing Only Tumor Cells
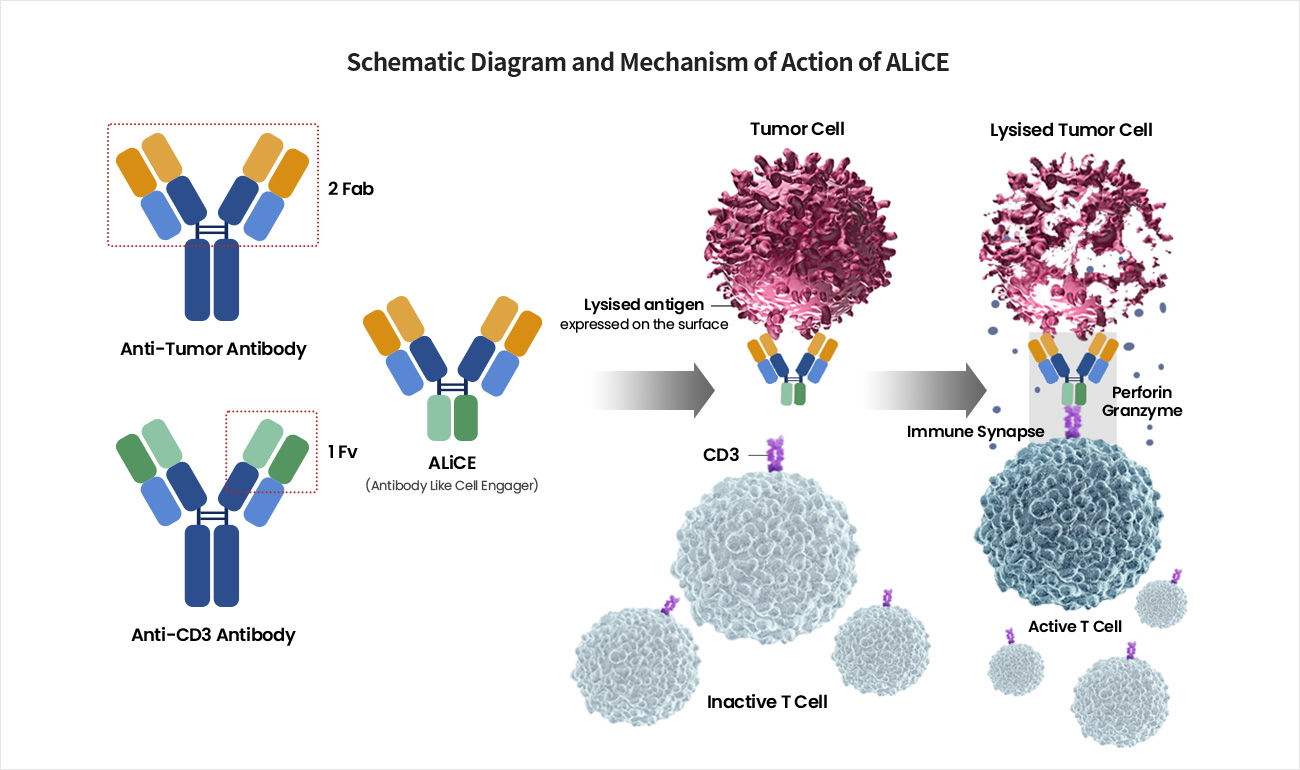
ALiCE first binds to the target antigen on the surface of cancer cells through high affinity, bivalent binding. ALiCE then binds to CD3 present on CD8+ cytotoxic T cells near the cancer cell, resulting in the formation of an immune synapse and stimulating the T cell to become activated to kill the cancer cell even without the TCR-MHC binding signal.
01
Proprietary Technology
- Powerful anticancer efficacy, while overcoming issues associated with existing anticancer drugs (short half-life in the blood, low productivity, and high toxicity)
- Patented in the United States in 2020 in recognition of its innovativeness
02
Bispecific Antibody Platform
- Bridges cancer cells and T cells together by simultaneously binding to a tumor associated antigen and CD3 on T cells
- Activates T cells to promote immediate killing of the cancer cells bound to the bispecific antibodies, lending to its stronger efficacy in comparison to existing immune checkpoint inhibitors
03
Human Antibody Structure-Inspired 2-by-1 Format
- Manufactured in a Y-shaped 2-by-1 format, similar to the structure of a human antibody, increasing antibody binding affinity to 50 to 100 times higher than that of existing CD3 targeted bispecific antibody formats
- When administered intravenously, the bispecific antibodies bind most of the cancer cells
04
ALiCE’s Optimal Molecular Weight
- Designed to have an optimal molecular weight to minimize excretion from the kidneys and ensure excellent stability in the bloodstream
- Stability in the bloodstream increases its circulating half-life, so patient discomfort can be improved dramatically by reducing dosage and infusion time
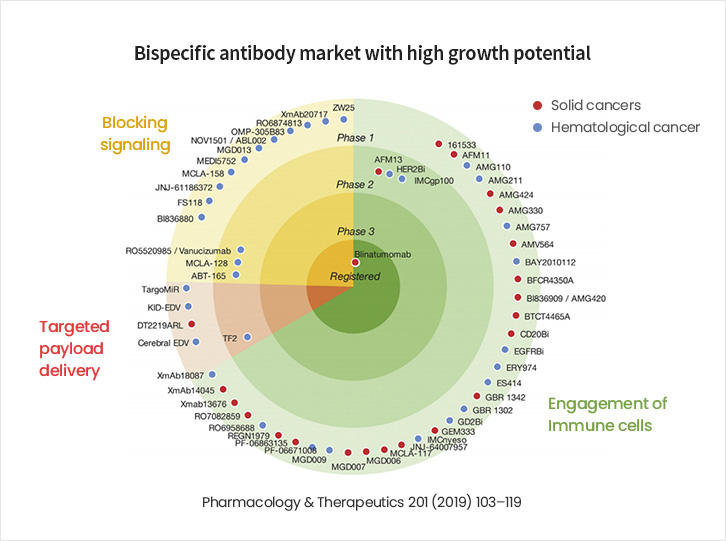
A Major Market, Expected to Grow to KRW 9 trillion in Size by 2030
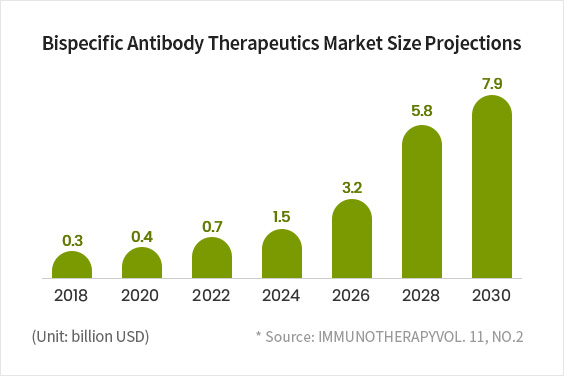
- Since the FDA approval of Amgen’s Bispecific T cell Engager (BiTE) in 2008, large global pharmaceutical companies have been joining the race to develop and commercialize bispecific antibodies.
- With expectations that bispecific antibodies will be more effective than monospecific antibodies, the bispecific antibody market is projected to grow from KRW 3.5 trillion in 2018 to more than KRW 9 trillion by 2030.
- Bispecific Antibody Market with High Growth Potential

- A look at the bispecific antibody pipelines for which clinical trials are currently being conducted shows that 49 (86%) of 57 substances are in Phase 1 (as of 2021).
- Thus, the bispecific antibody market demonstrates strong growth potential as early-stage therapeutics advance along the development pipeline.