Venturing into Immuno-Oncology Market with Antibody Library and Bispecific Antibody Platform
DATE : 2021.06.02Author : Y-Biologics
VIEWS : 1599
“We plan to proceed with an
expansion study on YBL-006, an immuno-oncology drug that has entered a Phase 1
clinical trial”
In response to the genetic engineering boom in the 1980s, LG Life Sciences (present-day LG Chem) established a genetic engineering research institute in Oakland. Park who was dispatched there returned to Korea in 1999 after mastering various genetic engineering techniques for 14 years and endeavored to develop new drug called antibodies. Since Janssen received marketing approval from the U.S. Food and Drug Administration (FDA) for Orthoclone, a therapeutic agent for kidney transplant rejection, in 1985, he was among the first in Korea to begin antibody drug research.
After LG Life Sciences shut down its antibody drug development operations, Park joined the Korea Research Institute of Bioscience and Biotechnology where he studied antibodies for about a decade before establishing a startup to develop immuno-oncology drugs. We met with Young Woo Park, the founder and CEO of Y-Biologics, who is preparing to take another leap forward for new drug development through an IPO by filing a preliminary review application to the Korea Exchange, to hear the story of Y-Biologics, a developer of antibody drugs.

Q. You were among the first in Korea to study antibody drugs. I am curious as to why you got into antibody research.
A. I got my first real job in 1985 at the LG Chem Research Center located in Jeonmin-dong, Daejeon. Back then, there was a boom of genetic engineering, and large corporations were establishing genetic engineering research institutes. So did LG Chem in Daejeon. In Korea, at the time, genetic engineering technology was not up to the standard, so LG established a local research institute in Oakland near San Francisco.
I returned to Korea around 1999 when LG commenced an antibody project. From 1997 to 2000, blockbuster antibody drugs were being developed, and LG also jumped in. Considering that drug development in Korea centered on small molecules at the time, it was a bold move.
I got to learn many new things while doing research on antibodies at LG and the Korea Research Institute of Bioscience and Biotechnology (KRIBB). What was certain was that it was worth giving a shot at building a library of antibodies, compared to small molecules, as long as one had excellent technology because the scale did not matter. So I came to the conclusion that even a startup could give a shot at this.
Q. That was a great segue, explaining why established a startup. Domestic startups focusing on development of single pipeline and new drug development models using antibody libraries sound somewhat new.
A. I have been conducting research using phage technology since the time I was doing my master’s. Based on that experience, I conducted molecular cloning research at LG. With the research experience I gained at LG and KRIBB, I was confident when it came to synthesizing antibodies.
Of course, in order to explain this to someone, a process of providing proof was necessary. So, to demonstrate, we went through the process of checking the diversity of the antibodies we had using next-generation sequencing (NGS) three to four years ago.
In other words, our antibody library exists in the form of DNA, and we checked the sequence diversity through an antibody phagemid sequencing process. The higher the antibody diversity, the better the library quality.
In this process, we were able to discover an excellent PD-1 antibody, YBL-006, for which a Phase 1 clinical trial is currently underway.
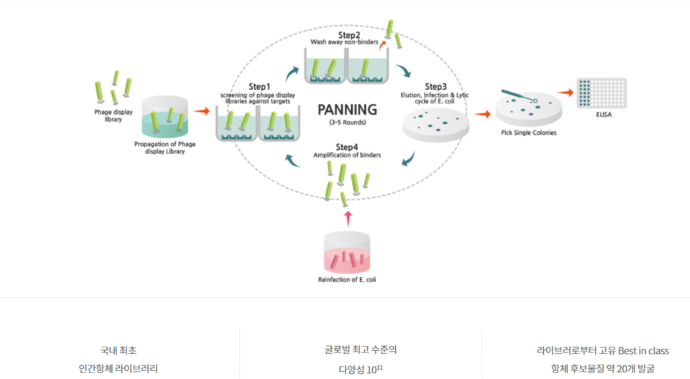
Y-Biologics’
antibody library, Ymax-ABL [Source: Y-Biologics website]
Q.
Please explain YBL-006 in detail.
A. The global PD-1 antibody market is currently worth KRW 30 trillion. The market is dominated by Keytruda (pembrolizumab) and Opdivo (nivolumab). We are conducting clinical research to get into this market. We have carried out a Phase 1 trial with 11 subjects, and we plan to increase the number of subjects to 50 through an expansion study.
In the case of the clinical trial with 11 subjects, it was a process of checking the safety in terminal cancer patients, irrespective of the type of cancer they had. Once the results from the expansion study with 50 subjects come out, we will present them at the European Society for Medical Oncology (ESMO).
Q. The PD-1 immuno-oncology drug (antibody) market is already dominated by Keytruda and Opdivo. What sets YBL-006 apart from others?
A. When the physical properties were tested during the preclinical process, it was found that the properties of YBL-006 were much superior. YBL-006 exhibited five times better binding affinity than Keytruda. The area where PD-1 and PD-L1 ligands are bound by YBL-006 is much larger compared to Keytruda, based on which we can expect high efficacy.
Q. You said that the Phase 1 clinical trial was conducted with 11 subjects with all kinds of cancer. What type of cancer will be targeted in the expansion study?
A. In order to speed up the development process, relatively speaking, we will target rare types of cancer. We have formed a scientific advisory board (SAB) with the CEO of Bang & Ok Consulting, Professor Eui Cheol Shin from KAIST Graduate School of Medical Science and Engineering, Professor Woo Ik Jang who has worked at CHA Hospital and Handok, and Professor Seung Hoon Lee who was at the National Cancer Center and is now at Daejeon Eulji Medical Center.
We are discussing the SAB about which the types of cancer we will target in our subsequent clinical trials. Since there are already similar drugs out on the market, the goal is to obtain approval as quickly as we can by demonstrating clear effectiveness. We signed an MOU with Samsung Medical Center in Seoul to pave the foundation for follow-up clinical trials. We are also discussing the matter with the National Cancer Center.
Q. You have partners for other pipelines but not for YBL-006. Do you have a specific timepoint to license out the technology in mind?
A. After the Phase 2 clinical trial, we will begin considering licensing out the technology. Also, research on combination therapy with the PD-1 antibody and other anticancer drugs is being carried out in full swing, so this will be our research strategy.
Q. It seems that such active collaborations have been possible since you have such an excellent antibody library. Could you explain your collaboration strategy?
A. CAR-T therapeutics, antibody-drug conjugates (ADCs), and T cell engagers are similar in that they are designed to control cancer cells. Ultimately, the key is to accurately target cancer cells. Antibodies play a crucial role in the target process. Once a suitable antibody is discovered, it ultimately becomes possible to effectively develop a CAR-T cell therapy agent, ADC, and T cell engager.
Although there have been active discussions about T cell engagers, they have yet to be used widely as a drug is because there are hurdles caused by the toxicity issue and so on. This can be addressed with antibodies. This is the reason we are conducting joint research with LegoChem Bioscience, Intocell, and AbTis, which have ADC-related technology.
Our company is equipped with a bispecific antibody platform called ALiCE and an antibody library called Ymax-ABL. In addition, we will file a patent registration application after developing a platform that can target and kill only tumor cells.

△Y-Biologics’
bispecific antibody platform, ALiCE [Source: Y-Biologics website
Q. So there can be a platform that can target and kill only tumor cells? I’d like to know more.
A. This is a strategy to leverage the activity of the antibody by using the tumor microenvironment (TME). Startups abroad have jumped into the platform development race, and big pharmaceutical companies are buying these technologies. Research for a more precise effector function in the TME is being carried out in full swing as well.
To be more specific, antibody activity can vary depending on the pH and proteases, which are enzymes that break down proteins, and the strategy is to pick out antibodies that can function in the relevant environment. The ultimate objective is to target only cancer cells without touching normal cells. These kinds of antibodies will be useful to companies that develop ADC and CAR-T therapeutics.
It was possible for us to develop this type of platform because we had the bispecific antibody platform, ALiCE, and the antibody library, Ymax-ABL."

Q. It may be too early to tell, but do you have a specific plan for raising capital after going public?
A. Based on solid preclinical research, we will pursue in-house development until at least Phase 1. Then, we will seek to license out the technologies afterwards. When it comes to antibodies, cell line development and sample production are very costly. We will reinvest the funds to further expand our pipelines.
Q. What are your ultimate aspirations as a startup specializing in new drug development?
A. I’ve heard that Keytruda or Opdivo are not accessible to all patients due to the limited health insurance coverage in Korea. These drugs are used in primary treatment for non-small cell lung cancer which is quite prevalent, so I understand the sufferings of the patients and the concerns of the authorities. I want to contribute to enhancing the accessibility of quality drugs for patients by obtaining approval for a domestically developed PD-1 antibody drug as soon as possible."
Source: Hit News (http://www.hitnews.co.kr)
