Y-Biologics Publishes Paper on Bispecific Antibodies Based on ALiCE Platform
DATE : 2021.04.05Author : Y-Biologics
VIEWS : 1240
Set to expand the pipelines and currently developing an upgraded version of ALiCE

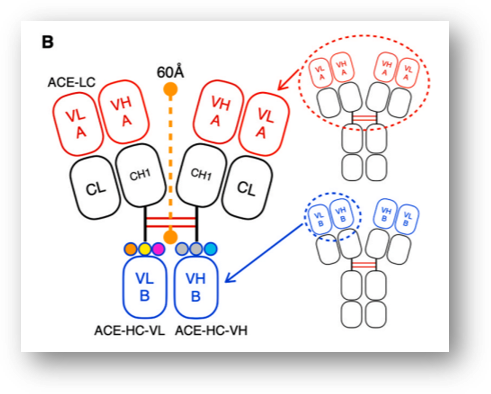
ALiCE is a bispecific antibody technology in which the Fc domain of the antibody is substituted with VH-VL, which recognizes a different antigen. It is about 140 kDa in size. Fab (VH-VL), the original bivalent antigen recognition site, was maintained, while VH-HL that recognizes another antigen was incorporated into the Fc domain. This has resulted in a novel bispecific antibody with a 2:1 design that recognizes two different antigens.
As for the background of coming up with this design, the first-generation bispecific T cell engager (BiTE, approx. 55 kDa) developed by Amgen has a short drug half-life, thus requiring ongoing injections and has had toxicity issues in activating T cells and application to normal tissue. In BiTE, VH and VL are connected at a ratio of 1:1.
Y-Biologics’ novel format is expected to have specificity for cancer tissues where there is high expression of cancer antigens compared to normal tissues, based on the characteristics of binding to cancer antigens and T cells in a 2:1 ratio.
In the published paper, Y-Biologics presents its research findings that showed the ALiCE platform, and YBL-013 (ACE-05), to which the ALiCE platform in an in vitro cancer cell model and in an in vivo mouse model, had lower toxicity and better ability to kill cancer cells compared to the BiTE platform. It was also confirmed that tumor growth was inhibited when YBL-013 was administered in a mouse model with humanized non-small cell lung cancer (HCC827+human PBMC).
In primates, the drug half-life was found to be higher than that of BiTE. In cynomolgus monkeys, for instance, the half-life of BiTE was 9.0 hours, whereas the half-life of YBL-013 was more than three times higher at 31 hours.
Bum Chan Park, the Head of Research at Y-Biologics, said, “This paper confirmed that our proprietary ALiCE platform has excellent competitiveness in the field of CD3-based bispecific T cell engagers. In addition to YBL-013, Y-Biologics has been discovering and developing a number of novel drug pipelines based on the ALiCE platform, and there are plans to further expand the pipelines. [...] Furthermore, we are in the stage of completing the upgrading process for the ALiCE platform to extend the half-life in the bloodstream, and we will apply it to target antigens of various types of solid tumor.”
Meanwhile, four patents have been registered for the ALiCE platform in the United States, and patent applications have been submitted in major countries such as Korea, Europe, China, and Japan.
Y-Biologics concluded a licensing agreement with 3D Medicines for the exclusive rights to the PD-L1xCD3 bispecific antibody, YBL-013, in China. Y-Biologics has recently signed a CDMO contract with Wuxi and is pursuing commercial development.